Abstract
Introduction: Myelodysplastic syndromes (MDS) are neoplasms characterized by cytopenias, high risk of leukemic progression, and poor overall survival. Chemotherapy for MDS is not curative, and no new drugs have been approved for the treatment of MDS in over a decade. Clinical trials should be considered at any time during the management of patients with MDS, but enrollment criteria may be barriers that limit accrual. In this study, we extracted MDS clinical trial data from clinicaltrials.gov, and compared study indications and characteristics, including inclusion and exclusion criteria.
Methods: We identified MDS clinical trials via clinicaltrials.gov (accessed: April 16, 2018). Studies were included if they allowed "MDS," "Myelodysplastic syndromes," "Preleukemia," and/or "Myelodysplasia," based on the pre-defined 'related terms' criteria in the database. We included interventional studies open in the United States that were listed as recruiting or not yet recruiting, for adults (age 18-64) and older adults (age 65+). We excluded studies that were observational in nature, involved a transplant-based intervention, or did not use a pharmacological intervention. We coded inclusion and exclusion criteria based on those provided by study authors in the database.
Results: 83 interventional clinical trials enrolling patients with MDS were identified. Studies started enrollment between 4/1/2013 and 3/27/2018, and anticipated reaching the primary objective between 5/1/2017 and 6/1/2025. The median planned study duration was 38 mo (range 10-95). In total, studies sought to enroll 8866 patients over 273 study-years; the median study enrollment estimate was 1.7 patients/month, or across all trials 247 patients/month. Clinical trials could be exclusive to MDS patients (n=28), include MDS patients and other myeloid malignancies e.g. AML (n=44), or include MDS patients and other cancers including solid tumors (n=11). For clinical trials exclusive to MDS, the total enrollment goal was 1966 patients over 96 study-years, with a median rate of 1.4 patients/month (range 0.3-13.2) or total of 63 patients/month across all studies.
33 trials were phase 1 studies, 17 were phase 1/2, 26 were phase II, 1 trial was phase 2/3, and 6 were phase III studies. The primary endpoint was typically MTD (n=50) or ORR (n=22), while 5 studies had an OS endpoint.
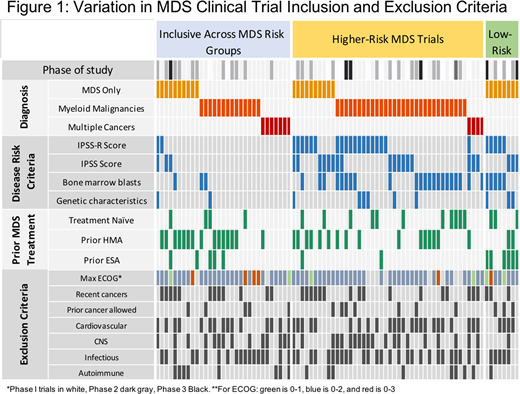
Most trials specified "higher risk" MDS (n=44); 8 specified "lower risk" MDS and 31 allowed all MDS risk or did not specify risk (Figure 1). Lower risk MDS studies were all exclusive to MDS patients. Inclusion criteria related to MDS risk varied significantly according to whether a study was MDS-specific or not (p=0.021): 82% of MDS-specific trials had risk exclusions, compared to 72% of myeloid trials, and only 36% of trials open across cancers. Of 52 trials specifying MDS risk, 20 included IPSS criteria, 24 included IPSS-R criteria, and 27 had blast count criteria.
Lower risk MDS criteria was variably defined as IPSS low or INT-1 disease (n=3), IPSS-R very low or low risk (n=1), IPSS-R VL, L, or intermediate risk (n=4), or blast counts < 5% (n=1), < 10% (n=2), or <20% (n=2). There were variations in the criteria for transfusion dependence, including 2 transfusion units in 8 weeks (n=3), 4 in 8 weeks (n=2), 2 in 4 weeks (n=2), 1 in 6 weeks (n=1), and 2 in 16 weeks (n=1).
For higher risk MDS, criteria included IPSS INT-1, INT-2, or High risk (n=7), IPSS INT-2 or High risk (n=12), IPSS-R intermediate, high, or very high risk (n=12), or IPSS-R high or very high (n=9); blast counts were set at >5% (n=12) or >10% blasts (n=12). Most studies specified exclusion of CNS disease, even though CNS involvement is exceptionally rare in MDS.
43 trials excluded concurrent cardiovascular disease; most often (n=18) requiring 6mo since a cardiovascular event. 46 trials had language excluding concurrent cancers, including 4 that did not allow any prior cancer, and 17 required ³24mo disease free. Exclusions for prior cancers did not vary according to primary outcome (MTD or PK, vs ORR/OS, p=0.16). 20 of 56 studies with MTD or early outcomes (e.g. PK) required cancer-free intervals of ³1y, while 7 allowed concurrent cancers if not on active therapy.
Discussion: Currently enrolling MDS clinical trials show significant variation in their inclusion and exclusion criteria. Heterogeneous definitions of basic entry criteria, such as the definition of higher- and lower-risk MDS, may cause barriers to enrollment.
Brunner:Takeda: Research Funding; Novartis: Research Funding; Celgene: Consultancy, Research Funding. Garcia:Celgene: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.